In a landmark medical achievement, a young man in New York has been reported as the first person in the state of New York to be cured of sickle cell disease, marking a major milestone in the long-standing battle against one of the world’s most debilitating genetic blood disorders.
This milestone comes as researchers and clinicians around the world steadily advance gene-based therapies, including CRISPR-mediated approaches and stem cell interventions, a shift that represents not just treatment, but long-term functional cures. In this article, we explore the current state of sickle cell research, how recent scientific advances are reshaping the field, and what this means for patients globally.
What Is Sickle Cell Disease?
Sickle cell disease (SCD) is a hereditary blood disorder characterized by abnormally shaped red blood cells that impair oxygen transport and lead to episodes of severe pain, organ damage, and reduced quality of life. The condition disproportionately affects individuals of African, Mediterranean, and Middle Eastern descent. Traditional treatments have focused on pain management, blood transfusions, and prevention of complications but a definitive cure has remained elusive for decades.
The New York Breakthrough: A Functional Cure
A Functional Cure: what it means clinically (no vaso-occlusive crises, transfusion independence, sustained Hemoglobin production), and that long-term follow-up is ongoing.
At Cohen Children’s Medical Center in New York, doctors successfully applied a novel therapy using the patient’s own bone marrow to regenerate healthy red blood cells that do not sickle, effectively providing a cure rather than symptom control. The therapy involved extracting the patient’s hematopoietic (blood-forming) stem cells, modifying them, and reinfusing them to produce normal, oxygen-carrying cells. https://www.wndu.com
“This is the first cure you are seeing,” said Dr. Jeffrey Lipton, a hematologist involved in the treatment, emphasizing the historical importance of the step forward.
The patient, who suffered from sickle cell since infancy, now reports freedom from chronic pain crises and significant improvement in daily life, from travel to basic physical activity.
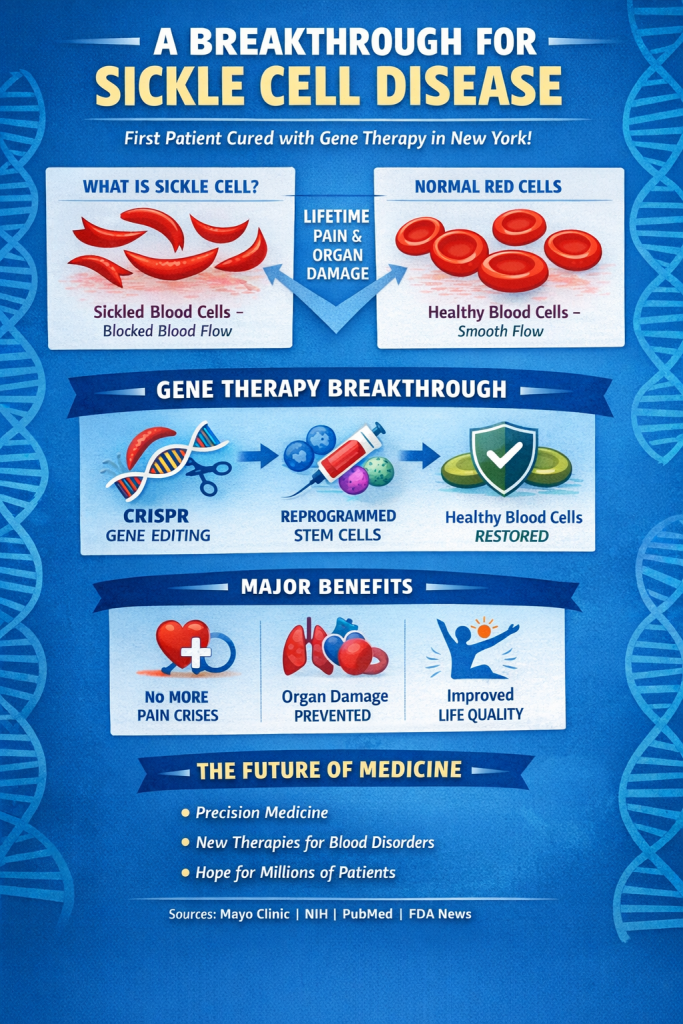
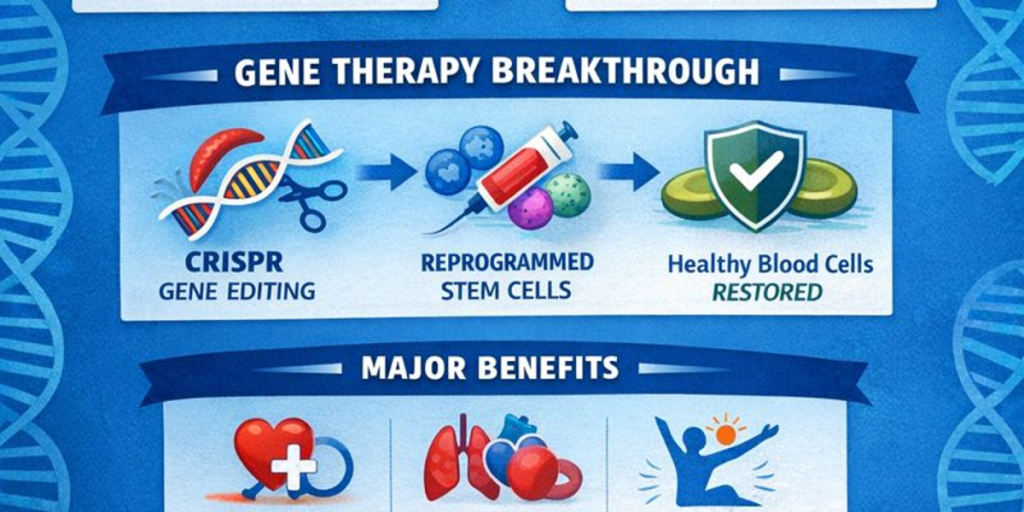
CRISPR and Gene Therapy: A Revolution in Medicine
What Is CRISPR?
CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) is a gene-editing technology that allows scientists to precisely alter DNA sequences within living cells. It has revolutionized genetics and holds promise for curing genetic diseases like sickle cell by correcting or disabling faulty genes. According to peer-reviewed research, CRISPR has enabled targeted modifications that correct gene defects at the root cause rather than merely treating symptoms. (Evidence and mechanism available via PubMed and multiple genomic research publications.)
Before CRISPR: The “Savior Sibling” Approach and the Roots of Curative Hematology
Before CRISPR-based gene editing reshaped the sickle cell landscape, hematology had already achieved curative intent through an extraordinary intersection of reproductive genetics, immunology, and transplantation science. One such approach, commonly referred to as the “savior sibling” strategy relied on in vitro fertilization (IVF) with preimplantation genetic testing and HLA typing to conceive a healthy, compatible sibling who could serve as a stem cell donor.
In families with multiple children affected by sickle cell disease, embryos were genetically screened to ensure the absence of the sickle mutation (HbSS) while simultaneously selecting for full HLA compatibility with an affected sibling. Upon birth, hematopoietic stem cells most commonly harvested safely from umbilical cord blood were used in an allogeneic hematopoietic stem cell transplant. The result was not symptom control, but durable hematologic reconstitution and functional cure.
This approach represented one of the earliest forms of precision medicine: tailoring reproduction, immunogenetics, and transplantation to rewrite a disease trajectory once considered irreversible.
FDA Approvals and Gene Therapy Advances
In recent years, the U.S. Food and Drug Administration (FDA) has approved multiple gene-based therapies for sickle cell disease that harness CRISPR strategies or related gene-editing platforms. These therapies have transformed SCD from a life-threatening condition into one that is manageable and potentially curable for many patients.
One notable treatment involves editing the patient’s own hematopoietic stem cells to produce fetal hemoglobin, which does not sickle and can effectively replace the defective adult hemoglobin.
- Exagamglogene autotemcel (Casgevy) = CRISPR-edited cells (BCL11A
enhancer) to raise fetal hemoglobin - Lovotibeglogene marcelpival (Lyfgenia) = lentiviral gene addition
Risks & Eligibility: What Patients Should Know
Gene-based therapies and curative-intent transplants can be life-changing, but they are not “simple injections.” Most current approaches require several steps and careful patient selection.
1) Conditioning chemotherapy is usually required
Before edited or donor stem cells are infused, patients often receive conditioning (myeloablative or reduced-intensity) chemotherapy to make space in the bone marrow. This can cause fatigue, nausea, mouth sores, low blood counts, and a temporary period of serious immune suppression.
2) Infection and bleeding risks during low blood counts
Because blood counts drop after conditioning, patients may face a period of increased infection risk and may require transfusions, hospitalization, and preventive antibiotics/antivirals depending on the protocol and center.
3) Fertility and infertility risk
Some conditioning regimens can impair fertility. Many patients are counseled to consider fertility preservation options (such as egg/embryo freezing or sperm banking) before treatment when feasible.
4) Long-term monitoring (including secondary malignancy surveillance)
Patients need long-term follow-up to monitor blood counts, organ function, durability of response, and rare but serious risks, including the possibility of secondary malignancies or other late effects. This is why many protocols include multi-year monitoring.
5) Eligibility and access barriers
Not everyone qualifies immediately. Eligibility often depends on disease severity (such as recurrent pain crises), organ function, and ability to tolerate conditioning. Access can also be limited by cost, insurance coverage, geography, and the availability of specialized centers with gene therapy programs.
Clinical note: This area is evolving quickly. Protocols vary by treatment type and center, and patients should discuss individualized risks with a hematologist at a qualified program.
A Personal Reflection
As a medical graduate, I hold deep admiration for clinicians who remain rooted in research—those who push medicine forward while many focus solely on practice. Scientific progress like this does not occur in isolation; it is built by geneticists, molecular biologists, embryologists, hemato -pathologists, clinical trial coordinators, nurses, and physician-scientists working in unison.
And there is one specialty I secretly , profoundly admire: hematology.
Hematologists do not merely study blood; they study the substrate of life itself.
Blood carries oxygen, nutrients, immune cells, clotting factors, hormones, signaling molecules, every system depends on it. To understand blood is to understand physiology at its most intricate level: immunology, molecular biology, genetics, metabolism, and systemic regulation converging in one fluid tissue.
To the hematology residents, fellows, attending physicians, and researchers: your work is foundational. Without blood, there is no endocrine signaling. No immune defense. No healing. No life.
Scientific Credibility: What the Research Shows
A vast body of research supports both the safety and efficacy of gene therapies for blood disorders:
PubMed-indexed clinical trials show that engineered stem cell therapies can yield sustained production of healthy blood cells.
Multiple studies highlight the ability of CRISPR-based edits to target and correct specific genetic mutations responsible for SCD.
Clinical research demonstrates improvements in patient quality of life, reduced pain crises, and decreased need for transfusions.
This strong scientific foundation underscores that what once seemed like science fiction now stands at the cutting edge of clinical practice.
What This Means for Patients and Families
For patients living with sickle cell disease, this milestone signifies:
- A Shift Toward Cures
Emerging therapies aim to correct the underlying genetic defect not just manage symptoms. - Reduced Long-Term Complications
Successful regenerative treatments have the potential to lower the risk of organ damage
and recurrent crises. - Expanding Access to Innovative Care
While initially available in specialized centers, gene therapy clinical programs are rapidly
expanding, guided by ongoing research and approvals.
Health organizations such as the CDC, NIH, and Mayo Clinic emphasize the importance of integrating these breakthroughs into broader treatment frameworks as evidence of efficacy grows.
Looking Ahead: The Future of Genetic Medicine
The progress in treating sickle cell disease is just one example of the broader transformation occurring in medicine thanks to gene editing and regenerative science. As more therapies emerge and clinical experience increases, the promise of precision medicine becomes more achievable for a wide range of genetic conditions.
This New York milestone not only represents one life changed — it affirms the potential of science to provide real, lasting cures for diseases once considered incurable.
What Comes Next? Access, Equity, and Global Impact
This breakthrough raises urgent questions:
Cost and Accessibility
Gene therapies currently cost hundreds of thousands to millions of dollars per patient.
Governments, insurers, and pharmaceutical companies must address pricing models, value-based reimbursement, and public-private partnerships.
Developing Countries
Sub-Saharan Africa bears the highest global burden of sickle cell disease. For these regions, solutions may include:
- Scaled gene-therapy manufacturing
- Regional treatment hubs
- Long-term investment in newborn screening and early intervention
- Technology transfer and workforce training
Policy and Ethics
Ensuring ethical distribution, long-term follow-up, and equitable access will define whether this breakthrough becomes a global solution or a privilege of wealth.
FAQ
Q1: Is sickle cell disease actually curable now?
For some patients, yes; curative-intent options include allogeneic stem cell transplantation and newer gene-based therapies that modify a patient’s own stem cells. Eligibility depends on disease severity, organ health, and access to a specialized center.
Q2: What does “functional cure” mean in sickle cell disease?
It typically means sustained remission such as elimination or major reduction of vaso-occlusive pain crises and improved hemoglobin function after a curative-intent therapy.
Ongoing long-term monitoring is still important.
Q3: How does CRISPR help treat sickle cell disease?
Some CRISPR-based strategies edit a patient’s stem cells to increase fetal hemoglobin or modify regulators that influence sickling. The edited cells are infused back after conditioning so they can produce healthier red blood cells over time.
Q4: What are the biggest risks of gene therapy for sickle cell disease?
Risks can include side effects from conditioning chemotherapy (infection risk, infertility risk), the need for hospitalization and transfusion support, and the requirement for long-term monitoring for rare late complications.
Q5: Who is most likely to qualify for these treatments?
Programs often prioritize people with severe disease (frequent pain crises or complications) who have organ function that can tolerate conditioning. Criteria vary by therapy, trial, and hospital center.
References & Further Reading
Man becomes first in New York to be cured of sickle cell anemia — WCBS News (2025) –https://www.wndu.com
PubMed – CRISPR gene editing clinical research abstracts – https://pubmed.ncbi.nlm.nih.gov/30482590/
https://www.mayoclinic.org/diseases-conditions/sickle-cell-anemia/symptoms-causes/syc-
20355876
https://pubmed.ncbi.nlm.nih.gov/39998298/
NIH/NCBI research on gene therapies and hemoglobinopathies